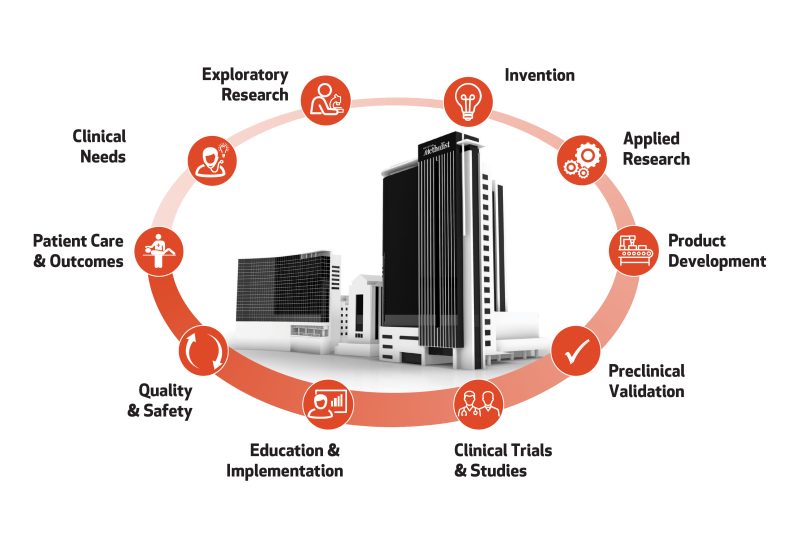
The Center for Rapid Device Translation is committed to collaborating with clients to navigate effective regulatory pathways through preclinical device testing. We assist with preparing the technologies, tools and facilities for applying Good Laboratory Practice (GLP), conducting successful safety studies and achieving robust data for regulatory agency approval. We guide our clients to opening the door to early phase clinical testing in humans.
As industry innovators, our clients have access to entrepreneurs and scientists with invaluable expertise and insight during the foundational planning and first phases of product development. We offer a full suite of services, centralized in the extensive research facilities of our research institute, enabling efficient workflow without leaving Houston Methodist, located in the heart of the Texas Medical Center (TMC). The TMC is the world’s largest medical complex, boasting eight academic and research institutions and 21 hospitals serving over 7 million visitors per year.
A Legacy of Innovation
The Houston Methodist legacy of innovation, exemplified by Dr. Michael E. DeBakey – who used his wife’s sewing machine to invent the Dacron graft in 1954 – is cultivated today through our translational research services. From the most advanced technologies to the most knowledgeable experts in meeting regulatory standards, we are empowered to forge new frontiers for advancing medicine.
Medical device development, prototyping and testing is a complex process. Our cGMP Manufacturing Core offers our clients a simpler route for producing a prototype and reaching the preclinical phases of development.